The Effects of Calcination Temperature of Nickel Supported on Candle Soot towards Ethanol Oxidation Reaction (EOR)
DOI:
https://doi.org/10.37934/jrnn.8.1.2330Keywords:
Calcination temperature, nickel, candle soot, ethanol oxidation reactionAbstract
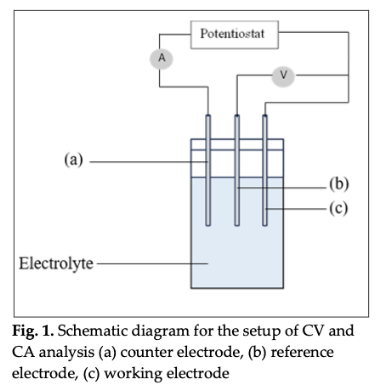
The discovery of cost-effective electrocatalyst materials suitable for anode catalyst for direct ethanol fuel cell (DEFC) is a significant problem. In this context, candle soot has been selected as a catalyst support material as it has higher electrical conductivity. Furthermore, there is a limited amount of research conducted on the use of candle soot as a catalyst support, making it an interesting and novel avenue of exploration. The purposes conducting this research was to evaluate the electrochemical properties of different calcination temperature supported on candle soot towards ethanol oxidation reaction (EOR). Ni/FS electrocatalyst were calcined at 600, 650, 700, 750, and 800 °C. Chronoamperometry (CA) and cyclic voltammetry (CV) methods were used to analyze the electrochemical characteristics. CV investigation showed that Ni/FS catalytic activity has the maximum current density of 6.93 mA/cm2 at 800 °C. 800 °C had the greatest IF/IB ratio (1.15) among the calcination temperatures. CA research showed that 800 °C Ni/FS, supported by candle soot, had the highest retention rate (50.00 %) and was the most stable electrocatalyst. Hence, 800 °C of calcination temperature is the maximum temperature for nickel supported on candle soot towards ethanol oxidation reaction.
Downloads