Nor Azwadi Che Sidik (azwadi@akademiabaru.com)
Aims and Scope
Journal of Advanced Research in Applied Sciences and Engineering Technology is an international forum for the publication and dissemination of original work which contributes to greater scientific understanding in the area of applied sciences and engineering technology. The Journals reports principally the achievements spanning the interdisciplinary field of applied researches. Scope of the journal includes: biology, chemistry, physics, environmental, health science, mathematics and statistics, geology, engineering, computer science, natural and technological sciences, medicine, and architecture.
Indexing


Beginning 2021, Journal of Advanced Research in Applied Sciences and Engineering Technology is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
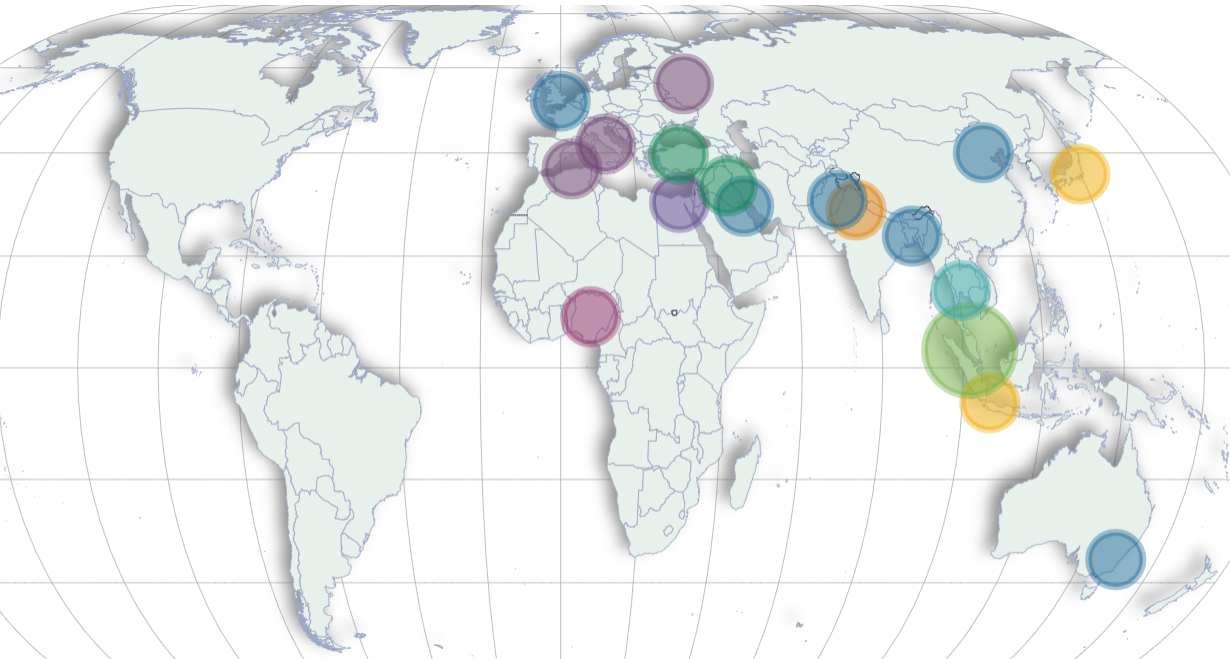
Distribution of authors