Synthesis and Evaluation of Fe-Doped Zinc Oxide Photocatalyst for Methylene Blue and Congo Red Removal
DOI:
https://doi.org/10.37934/progee.22.1.1328Keywords:
Zinc oxide photocatalyst, Doping, Photocatalytic degradation, Band gap energy, Sol-gel methodAbstract
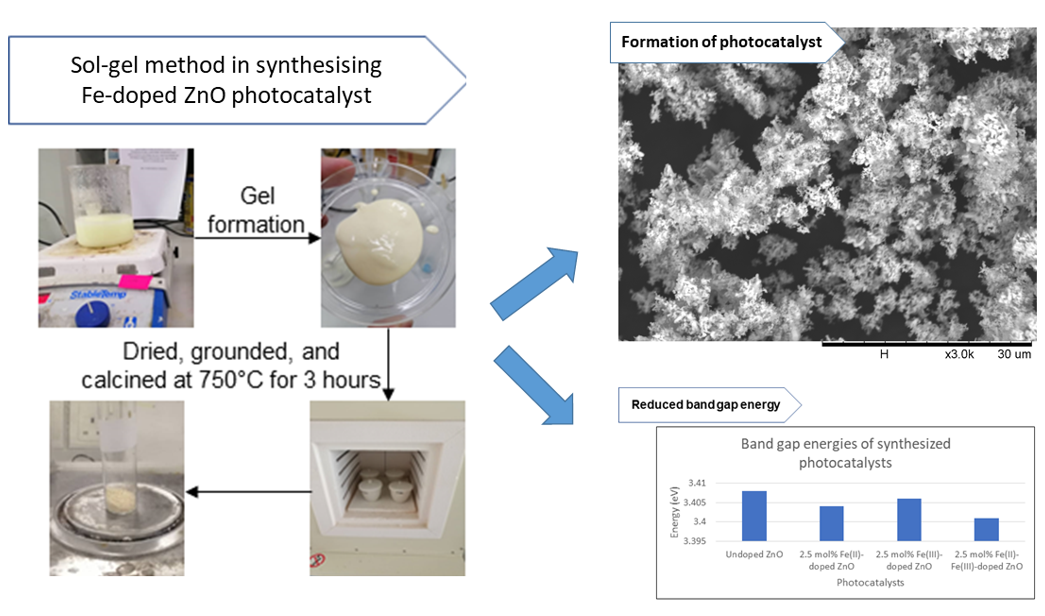
Zinc oxide is one of the most common photocatalysts utilized for the photocatalytic degradation of synthetic dyes aside from titanium dioxide. However, the application of ZnO in the treatment of wastewater containing synthetic dyes is limited due to the high energy band gap which allows ZnO to be efficient upon irradiation with ultraviolet radiation only. This study aims to evaluate the photocatalytic degradation efficiency of the zinc oxide photocatalyst and its derivatives, specifically 0.25, 0.5, 2.5 and 5 mol% Fe(II)-doped ZnO, 0.25, 0.5, 2.5 and 5 mol% Fe(III)-doped ZnO and 2.5 mol% Fe(II)-Fe(III)-doped ZnO. The performance of the photocatalysts was evaluated based on the effect of solution pH, effect of photocatalyst loading and nature of dye. The synthesis of photocatalysts were done using sol-gel synthesis method, and photodegradation tests were carried out under visible light exposure for 60 minutes. The photocatalysts were characterized with SEM, FTIR, and UV-Vis spectroscopy. The optical characterization results show that 2.5 mol% Fe(II)-Fe(III)-doped ZnO has the lowest band gap energy of 3.401 eV which was estimated using Tauc’s plot. This further validated the degradation performance of the 2.5 mol% Fe(II)-Fe(III)-doped ZnO photocatalyst where it displayed the highest photocatalytic degradation efficiencies at all pH and photocatalyst loading. The highest degradation achieved using methylene blue was 94.21% and 32.97% using congo red as model solute at optimum pH and 300 mg/L photocatalyst loading. In overall, the present study has proven that Fe-doped photocatalysts have the potential for the degradation of various synthetic dyes upon irradiation with visible light.
References
A. Ayele, D. Getachew, M. Kamaraj and A. Suresh, Phycoremediation of synthetic dyes: An effective and eco-Friendly algal technology for the dye abatement, Journal of Chemistry (2021) 9923643. https://doi.org/10.1155/2021/9923643.
R. Islam, Water pollution due to textile industry, (2020). https://www.textiletoday.com.bd/water-pollution-due-textile-industry/ (accessed February 25, 2022).
Z.Y. Velkova, G.K. Kirova, M.S. Stoytcheva and V.K. Gochev, Biosorption of Congo Red and methylene blue by pretreated waste streptomyces fradiae biomass - Equilibrium, kinetic and thermodynamic studies, Journal of the Serbian Chemical Society 83 (2018) 107–120. https://doi.org/10.2298/JSC170519093V.
Y. Kayabasi, Methylene blue and its importance in medicine, Demiroglu Science University Florence Nightingale Journal of Medicine 6 (2020) 136–145. https://doi.org/10.5606/fng.btd.2020.25035.
A. Stelmach-Goldys, M. Zaborek-lyczba, J. Lyczba, B. Garus, M. Pasiarski, P. Mertowska, P. Malkowska, R. Hrynkiewicz, P. Niedzwiedzka-Rystwej and E. Grywalska, Physiology, Diagnosis and treatment of cardiac light chain amyloidosis, Journal of Clinical Medicine 11 (2022) 911. https://doi.org/10.3390/JCM11040911.
I. Khan, K. Saeed, I. Zekker, B. Zhang, A.H. Hendi, A. Ahmad, S. Ahmad, N. Zada, H. Ahmad, L.A. Shah, T. Shah and I. Khan, Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation, Water (Basel). 14 (2022) 242. https://doi.org/10.3390/w14020242.
N. Kaur, J. Kaushal, P. Mahajan and A.L. Srivastav, Design of hydroponic system for screening of ornamental plant species for removal of synthetic dyes using phytoremediation approach, (2022). https://doi.org/10.21203/rs.3.rs-1301660/v1.
M.A. Abu-Dalo, S.A. Al-Rosan and B.A. Albiss, Photocatalytic degradation of methylene blue using polymeric membranes based on cellulose acetate impregnated with zno nanostructures, Polymers (Basel) 13(19) (2021) 3451. https://doi.org/10.3390/polym13193451.
G. Ren, H. Han, Y. Wang, S. Liu, J. Zhao, X. Meng and Z. Li, Recent advances of photocatalytic application in water treatment: A review, Nanomaterials 11(7) (2021) 1804. https://doi.org/10.3390/nano11071804.
C. Regmi, B. Joshi, S.K. Ray, G. Gyawali, R.P. Pandey, Understanding Mechanism of Photocatalytic Microbial Decontamination of Environmental Wastewater, Frontiers in Chemistry 6 (2018) 33. https://doi.org/10.3389/fchem.2018.00033.
R. del Angel, J.C. Durán-Álvarez and R. Zanella, TiO2-Low band gap semiconductor heterostructures for water treatment using sunlight-driven photocatalysis, in: Titanium Dioxide - Material for a Sustainable Environment, InTech, 2018. https://doi.org/10.5772/intechopen.76501.
N. Kamarulzaman, M.F. Kasim and R. Rusdi, Band gap narrowing and widening of ZnO nanostructures and doped materials, Nanoscale Research Letters 10 (2015) 346. https://doi.org/10.1186/s11671-015-1034-9.
B. Albiss and M. Abu-Dalo, Photocatalytic degradation of methylene blue using zinc oxide nanorods grown on activated carbon fibers, Sustainability 13(9) (2021) 4729. https://doi.org/10.3390/su13094729.
A.B. Mapossa, W. Mhike, J.L. Adalima and S. Tichapondwa, Removal of organic dyes from water and wastewater using magnetic ferrite-based titanium oxide and zinc oxide nanocomposites: A review, Catalysts 11(12) (2021) 1543. https://doi.org/10.3390/catal11121543.
E. Cerrato, C. Gionco, I. Berruti, F. Sordello, P. Calza, M.C. Paganini, Rare earth ions doped ZnO: Synthesis, characterization and preliminary photoactivity assessment, Journal of Solid State Chemistry 264 (2018) 42-47. https://doi.org/10.1016/j.jssc.2018.05.001.
M. Carofiglio, S. Barui, V. Cauda and M. Laurenti, Doped zinc oxide nanoparticles: Synthesis, characterization and potential use in nanomedicine, Applied Sciences 10(15) (2020) 5194. https://doi.org/10.3390/app10155194.
A.B. Lavand and Y.S. Malghe, Synthesis, characterization and visible light photocatalytic activity of carbon and iron modified ZnO, Journal of King Saud University – Science 30 (2018) 65-74. https://doi.org/10.1016/J.JKSUS.2016.08.009.
M.C. Paganini, A. Giorgini, N.P.F. Gonçalves, C. Gionco, A. Bianco Prevot and P. Calza, New insight into zinc oxide doped with iron and its exploitation to pollutants abatement, Catalysis Today 328 (2019) 230-234. https://doi.org/10.1016/j.cattod.2018.10.054.
K.A. Isai and V.S. Shrivastava, Photocatalytic degradation of methylene blue using ZnO and 2%Fe–ZnO semiconductor nanomaterials synthesized by sol–gel method: a comparative study, SN Applied Sciences 1 (2019) 1247. https://doi.org/10.1007/s42452-019-1279-5.
A. Razani, A.H. Abdullah, A. Fitrianto, N.A. Yusof, U.I. Gaya, Sol-gel synthesis of Fe2O3-doped TiO2 for optimized photocatalytic degradation of 2,4- dichlorophenoxyacetic acid, Oriental Journal of Chemistry 33 (2017) 1959–1968. https://doi.org/10.13005/OJC/330442.
S.F. Mousavi, F. Davar, M.R. Loghman-Estarki, Controllable synthesis of ZnO nanoflowers by the modified sol–gel method, Journal of Materials Science: Materials in Electronics 27 (2016) 12985–12995. https://doi.org/10.1007/s10854-016-5437-x.
A. Gnanaprakasam, V.M. Sivakumar and M. Thirumarimurugan, Influencing parameters in the photocatalytic degradation of organic effluent via Nanometal Oxide Catalyst: A review, Indian Journal of Materials Science 2015 (2015) 1–16. https://doi.org/10.1155/2015/601827.
R.P. Pal Singh, I.S. Hudiara and S.B. Rana, Effect of calcination temperature on the structural, optical and magnetic properties of pure and Fe-doped ZnO nanoparticles, Materials Science- Poland 34 (2016) 451–459. https://doi.org/10.1515/msp-2016-0059.
S. Babel, H. Sudrajat and A.A. Abraham, Manganese-and iron-doped zinc oxide for photocatalytic degradation of recalcitrant dyes, Thammasat International Journal of Science and Technology 21(4) (2016) 33-43. https://doi.org/10.14456/tijsat.2016.28.
J.Z. Lian, C.T. Tsai, S.H. Chang, N.H. Lin and Y.H. Hsieh, Iron waste as an effective depend on TiO2 for photocatalytic degradation of dye waste water, Optik 140 (2017) 197–204. https://doi.org/10.1016/J.IJLEO.2017.04.023.
V.M. Thanh, N.T. Huong, D.T. Nam, N.D.T. Dung, le Van Thu and M.T. Nguyen-Le, Synthesis of ternary Fe3O4/ZnO/chitosan magnetic nanoparticles via an ultrasound-assisted coprecipitation process for antibacterial applications, Journal of Nanomaterials 2020 (2020) 8875471. https://doi.org/10.1155/2020/8875471.
S. Roy, M.P. Ghosh, S. Mukherjee and P. Ghosh, Introducing the magnetic properties in Fe doped ZnO nanoparticles for spintronics application, (2021). https://doi.org/10.21203/rs.3.rs-180286/v1.
P. Makula, M. Pacia and W. Macyk, How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV-Vis spectra, Journal of Physical Chemistry Letters 9 (2018) 6814–6817. https://doi.org/10.1021/acs.jpclett.8b02892.
G. Yentür and M. Dukkanci, Synergistic effect of sonication on photocatalytic oxidation of pharmaceutical drug carbamazepine, Ultrasonic Sonochemistry 78 (2021) 105749. https://doi.org/10.1016/j.ultsonch.2021.105749.
N.N. Yunus, F. Hamzah, M.S. So'Aib and J. Krishnan, Effect of catalyst loading on photocatalytic degradation of phenol by using N, S Co-doped TiO2, IOP Conference Series: Materials Science and Engineering 206 (2016) 012092. https://doi.org/10.1088/1757-899X/206/1/012092.
H. Yang, J. Fan, C. Zhou, R. Luo, H. Liu, Y. Wan, J. Zhang, J. Chen, G. Wang, R. Wang and C. Jiang, Co3O4@CdS hollow spheres derived from ZIF-67 with a high phenol and dye photodegradation activity, ACS Omega 5 (2020) 17160–17169. https://doi.org/10.1021/acsomega.0c01131.
T. Munir, M. Latif, A. Mahmood, A. Malik and F. Shafiq, Influence of IP-injected ZnO-nanoparticles in Catla catla fish: hematological and serological profile, Naunyn-Schmiedeberg's Archives of Pharmacology 393 (2020) 2453–2461. https://doi.org/10.1007/s00210-020-01955-6.
P.J. Lu, W.E. Fu, S.C. Huang, C.Y. Lin, M.L. Ho, Y.P. Chen and H.F. Cheng, Methodology for sample preparation and size measurement of commercial ZnO nanoparticles, Journal of Food and Drug Analysis 26 (2018) 628–636. https://doi.org/10.1016/j.jfda.2017.07.004.
H. Sutanto, I. Alkian, M. Mukholit, A.A. Nugraha, E. Hidayanto, I. Marhaendrajaya and P. Priyono, Analysis of Fe-doped ZnO thin films for degradation of rhodamine b, methylene blue, and Escherichia coli under visible light, Materials Research Express 8 (2021) 116402. https://doi.org/10.1088/2053-1591/ac33fe.
K.V.A. Kumar, B. Lakshminarayana, T. Vinodkumar and C. Subrahmanyam, Cu-ZnO for visible light induced mineralization of Bisphenol-A: Impact of Cu ion doping, Journal of Environmental Chemical Engineering 7 (2019) 103057. https://doi.org/10.1016/j.jece.2019.103057.
A. Kumar, A review on the factors affecting the photocatalytic degradation of hazardous materials, Material Science & Engineering International Journal 1(3) (2017) 106-114. https://doi.org/10.15406/mseij.2017.01.00018.
M. Boumediene, H. Benaïssa, B. George, S. Molina and A. Merlin, Effects of pH and ionic strength on methylene blue removal from synthetic aqueous solutions by sorption onto orange peel and desorption study, Journal of Materials and Environmental Science 9 (2018) 1700–1711. https://doi.org/10.26872/jmes.2018.9.6.190.
N. Bel, H. Mohamed, S. Ouni, M. Bouzid, M. Bouzidi and A. Bonilla-Petriciolet, Adsorption and photocatalytic degradation of an industrial azo dye using colloidal semiconductor nanocrystals, (2022). https://doi.org/10.21203/rs.3.rs-1057236/v2.
S.R. Sowmya, G.M. Madhu and M. Hashir, Studies on Nano-Engineered TiO2 Photo Catalyst for Effective Degradation of Dye, IOP Conference Series: Materials Science and Engineering 310 (2018) 012026. https://doi.org/10.1088/1757-899X/310/1/012026.
N.B. Swan and M.A.A. Zaini, Adsorption of Malachite green and congo red dyes from water: Recent progress and future outlook, Ecological Chemistry and Engineering S 26 (2019) 119–132. https://doi.org/10.1515/eces-2019-0009.
H.R. Sousa, L.S. Silva, P.A.A. Sousa, R.R.M. Sousa, M.G. Fonseca, J.A. Osajima and E.C. Silva-Filho, Evaluation of methylene blue removal by plasma activated palygorskites, Journal of Materials Research and Technology 8 (2019) 5432–5442. https://doi.org/10.1016/j.jmrt.2019.09.011 .
K. Litefti, M.S. Freire, M. Stitou and J. González-Álvarez, Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark, Scientific Report 9 (2019) 16530. https://doi.org/10.1038/s41598-019-53046-z.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Progress in Energy and Environment

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.











